Welcome to Gaiacore Pharma
Gaiacore Pharma is a trusted WHO-GMP certified pharmaceutical company. It is our endeavour at Gaiacore Pharma to manufacture the purest & most potent formulations and make our product available to Patients across the globe. We use only superior Quality (USP/BP) Active Pharmaceutical Ingredients (APIs) and have set up a strict set of processes to ensure that all our products meet the highest quality standards in the industry.
We engage in Research & Development, Manufacturing, Sourcing, Marketing and Distribution of highest-quality pharmaceutical products. We are a fully integrated company with in-house Business Development, Research & Development, Manufacturing, and Regulatory compliance capabilities.
With the assistance of the latest equipment and technology, our automated and modern production processes have been prepared. We offer a complete range of products with prompt and reliable assistance for all our consumers.
Consequently, our large-scale production capacities, highly qualified production and quality control staff combine to give a perfect environment for manufacturing pharmaceutical products of the highest efficacy and quality. This synergy allows us to provide the said products to our clients in a timely manner that is extremely competitive.
All members of our organization are fully committed to quality and compliance, to ensure our products meet the highest quality standards.
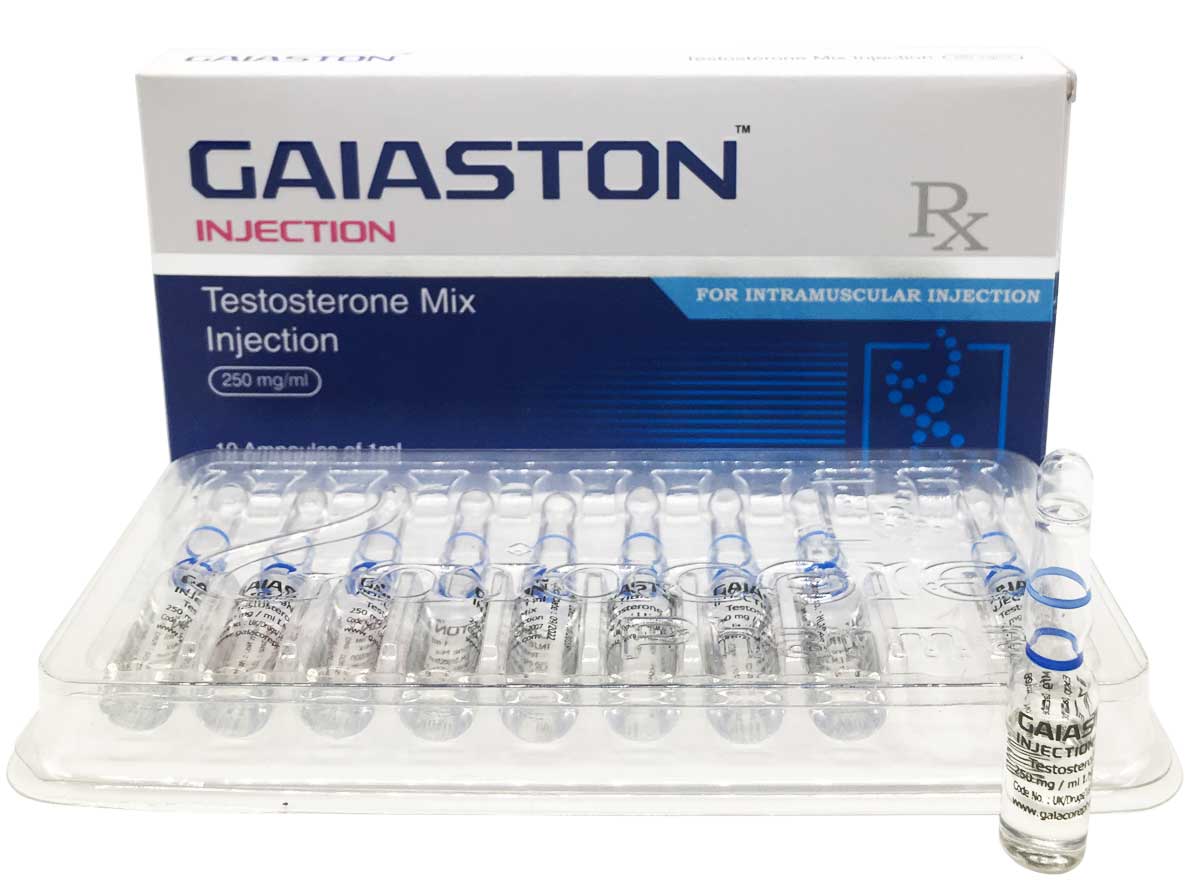
Gaiaston
Composition:
Each ml contains:
Testosterone Propionate USP 50mg
Testosterone Isocaproate BP 50mg
Testosterone Decanoate BP 100mg
Testosterone Phenylpropionate BP 50mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
PHARMACOLOGICAL CLASSIFICATION:
Pharmacotherapeutic group: Androgens. ATC code G03B A03
Treatment of hypogonadal men with Gaiaston results in a clinically critical ascent of plasma convergences of testosterone, dihydrotestosterone, estradiol and androstenedione, just as decline of SHBG (Sex hormone restricting globulin). Luteinizing hormone (LH) and follicle-invigorating hormone (FSH) are reestablished to the ordinary range. In hypogonadal men, treatment with Gaiaston results in an improvement of testosterone inadequacy indications. Additionally, treatment expands bone mineral thickness and fit weight, and diminishes muscle to fat ratio mass. Treatment additionally improves sexual capacity, including drive and erectile capacity. Treatment diminishes serum LDL-C, HDL-C and triglycerides and expands hemoglobin and haematocrit, which may prompt polycythaemia. No clinically pertinent changes in liver compounds and PSA have been accounted for. Testosterone likewise creates foundational impacts, for example, expanding the maintenance of sodium, potassium and chloride prompting an expansion in water maintenance. Treatment may result in an expansion in prostate size, and compounding of lower urinary tract manifestations, however no unfavorable impacts on prostate side effects have been watched. In hypogonadal diabeteic patients, improvement of insulin affectability as well as decrease in blood glucose have been accounted for with the utilization of androgens. In young men with sacred deferral of development and adolescence, treatment with Gaiaston quickens development and instigates improvement of auxiliary sex attributes. In female-to-male transsexuals, treatment with Gaiaston initiates masculinisation.
Pharmacokinetics:
Gaiaston contains four esters of testosterone with various spans of activity. The esters are hydrolysed into the regular hormone testosterone when they enter the general course.
Assimilation:
A solitary portion of Gaiaston prompts an expansion of complete plasma testosterone with pinnacle dimensions of around 70nmol/l (Cmax), which are come to roughly 24-48 h (tmax) after organization. Plasma testosterone levels come back to the lower furthest reaches of the ordinary range in guys in around 21 days.
In female-to-male transsexuals, a solitary portion of GAIASTON rehashed like clockwork brought about mean trough testosterone levels towards the upper end of the ordinary male range at 2, 4 and a year.
Conveyance:
Testosterone shows a high (over 97%) non-explicit official to plasma proteins and sex hormone restricting globulin in vitro tests.
Biotransformation:
Testosterone is processed to dihydrotestosterone and estradiol, which are additionally utilized by means of the typical pathways.
Disposal:
Discharge primarily happens by means of the pee as conjugates of etiocholanolone and androsterone.
Signs:
Testosterone trade treatment for male hypogonadism, when testosterone insufficiency has been affirmed by clinical highlights and biochemical tests. Testosterone organization may likewise be utilized as steady treatment for female-to-male transsexuals.
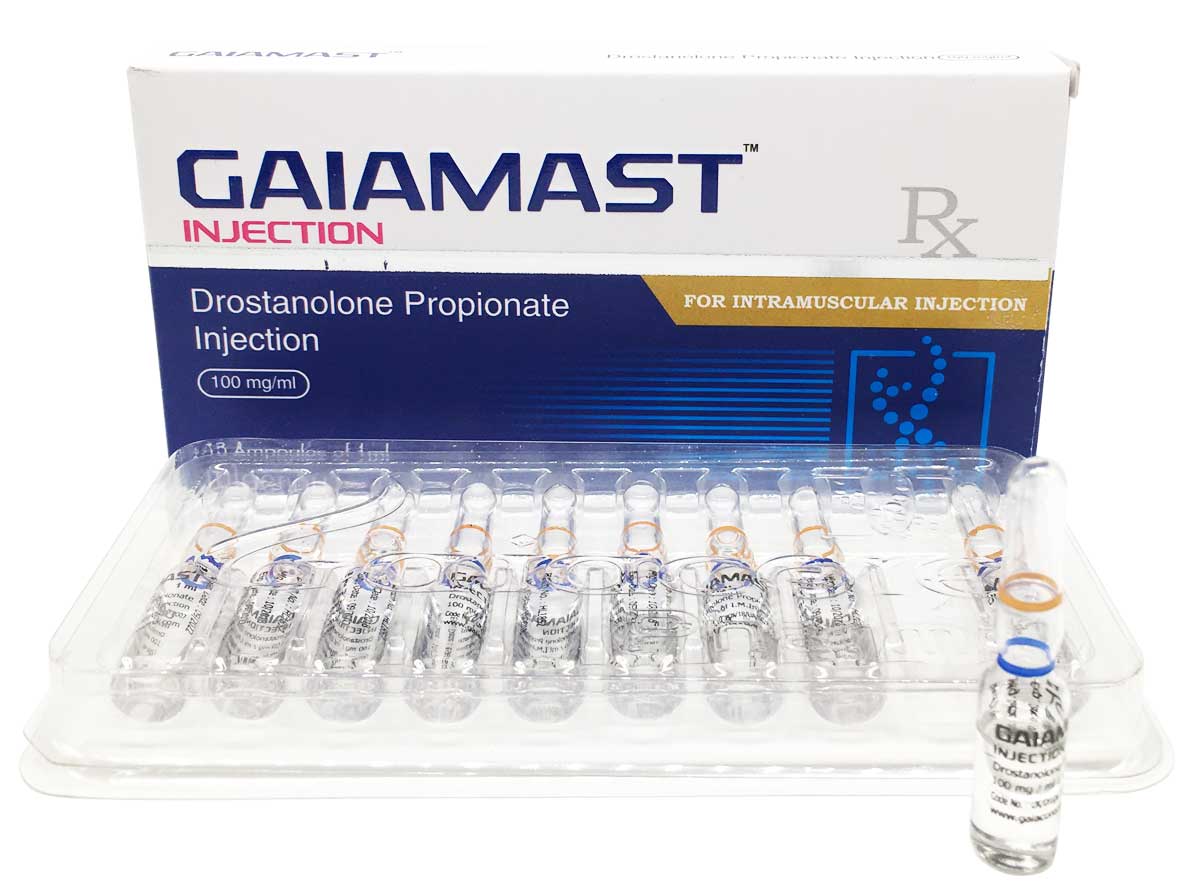
Gaiamast
Composition:
Each ml contains:
Drostanolone Propionate 100mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Indications and usage:
For use in females, for whitewashing of androgenresponsive repetitive mammary malignant growth in ladies who are over one year however under five years postmenopausal
Medication Interactions:
Androgens may build affectability to oral anticoagulants. Measurement of the anticoagulant may require decrease all together to maintain agreeable restorative hypoprothrombinernia. Simultaneous organization of oxyphenbutazone and androgens may result in raised serum dimensions of oxyphenbutazone. In diabetic patients. the metabolic impacts of androgens may diminish blood glucose and along these lines, insulin necessities.
Overdosage:
Incessant ingestion of high portions of anabolic steroids can cause heights in circulatory strain, left ventricular hypertrophy and premature coronary course infection.
Safety measures/Warnings:
Ladies create indications of virilism, with expanded facial hair, male example sparseness, skin inflammation, extending of the voice, sporadic menses and clitoral growth.
Changes in the larynx in ladies brought about by anabolic steroids can result in a rough, profound voice. The progressions are irreversible. Sodium and water maintenance can happen, and result in oedema; hypercalcaemia is likewise revealed. Androgen ingestion by a pregnant mother can cause virilization of a female baby.
Insulin obstruction with a fall in:
Glucose resistance and hypercholesterolaemia with a fall in high thickness lipoprotein cholesterol have been accounted for.
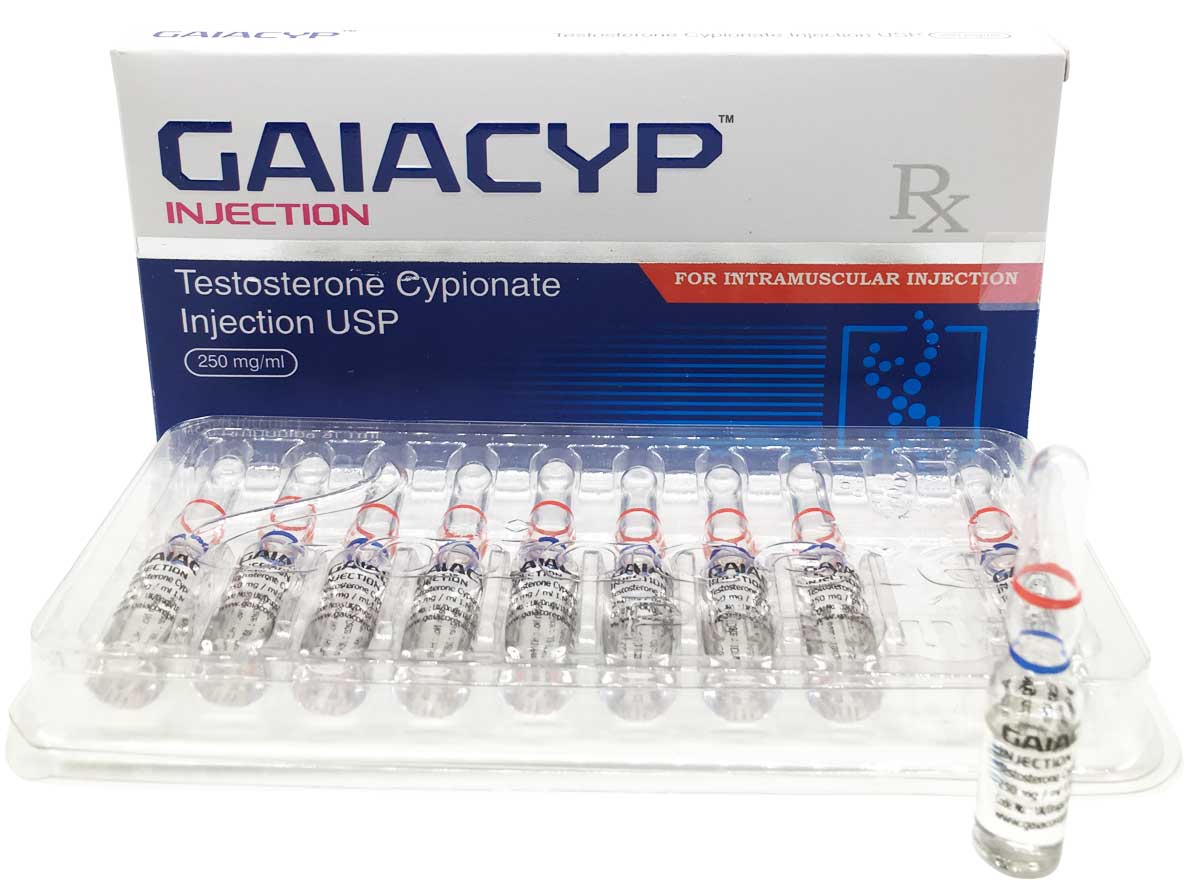
Gaiacyp
Composition:
Each ml contains:
Testosterone Cypionate USP 250mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Indications and usage:
Gaiacyp is indicated for replacement therapy in the male in conditions associated with symptoms of deficiency or absence of endogenous testosterone.
-
Primary hypogonadisrn (congential or acquired-testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, or orchidectomy.)
-
Hypogonadotropic hypogonadism (congenital or acquired)-idiopathic gonadotropin or LHRH deficiency, or pituitary-hypothalamic injury from tumours, trauma, or radiation.
Drug Interactions:
Androgens may increase sensitivity to oral anticoagulants. Dosage of the anticoagulant may require reduction in order to maintain satisfactory therapeutic hypoprothrombinemia. Concurrent administration of oxyphenbutazone and androgens may result in elevated serum levels of oxyphenbutazone. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and therefore insulin requirements.
Overdosage:
There have been no reports of acute overdosage with the androgens.
Precautions:
General: Patients with benign prostatic hypertrophy may develop acute urethral obstruction. Priapism or excessive sexual stimulation may develop.
Oligospermia may occur after prolonged administration or excessive dosage. If any of these effects appear, the androgen should be stopped and if restarted, a lower dosage should be utilized. Gaiacyp should not be used interchangeably with testosterone propionate because of differences in duration of action. Gaiacyp is not for intravenous use. Information for patients: Patients should be instructed to report any of the following nausea, vomiting. changes in skin color, ankle swelling, too frequent or persistent erections of the penis.
Laboratory tests: Hemoglobin and hematocrit levels (to detect polycythemia) should be checked periodically in patients receiving long-term androgen -administration. Serum cholesterol may increase during androgen therapy. Drug/Laboratory test Interferences: Androgens may decrease levels of thyroxine-binding globulin, resuhng in decreased total T4 serum levels and increased resin uptake of T3 and T4. Free thyroid hormone levels remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
Carcinogenesis : There are rare reports of hepatocellular carcinoma in patients receiving long term therapy with androgens in high doses. Withdrawal of the drugs did not lead to regression of the tumours in all cases. Geriatric patients treated with androgens may be at an increased risk of developing prostatic hypertrophy and prostatic carcinoma although conclusive evidence to support this concept is lacking.
Pregnancy: Teratogenic Effects. Pregnancy Category X.
Nursing mother: Gaiacyp is not recommended for use in nursing mothers.
Gaiaprop
Composition:
Each ml contains:
Testosterone Propionate USP 100mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
PHARMACOLOGICAL CLASSIFICATION:
Mechanism of Action
Testosterone Propionate: It is a highly anabolic as well as androgenic steroid. It is a common oil-based injectable testosterone. The added propionate extends the activity of the testosterone but it is still comparatively much faster acting than other testosterone esters such as cypionate and enanthate. Propionate is most commonly injected at least every third day to keep blood levels steady. This drug is quite effective for strength and muscle mass gains. Propionate is often very painful injection.
Clinical Pharmacology:
Endogenous androgen are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include growth and maturation of prostate, seminal vesicles, penis and scrotum, development of male hair distribution such as beard, pubic, chest and axillary hair, laryngeal enlargement, vocal chord thickening, alterations in body musculature and fat distribution.
Pharmacokinetics
Testosterone esters less polar than free testosterone. Testosterone esters in oil injected intramuscularly are absorbed slowly from the lipid phase, thus testosterone enantate can be given at intervals of two to four weeks.Testosterone in plasma is 98% bound to a specific testostrone estradiol binding globulin and about 2 % is free. The free testostrone concentrate will determine its half life. About 90% of a dose of testosterone is excreted in the urine as glucoronic and sulfuric acid conjugates of testosterone and its metabolites, about 6 % of a dose is excreted in the feces, mostly in the unconjugated form.
INDICATIONS:
Testosterone Propionate is indicated for replacement therapy in the male in conditions associated with symptoms of deficiency or absence of endogenous testosterone.
-
Primary hypogonadism (Congential or acquired) - testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, or orchidectomy.
-
Hypogonadotropic hypogonadisrn (congenital or acquired)-idiopathic gonadotropin or LHRH deficiency, or pituitaryhypothalamic injury from tumours, trauma, or radiation.
Gaiadeca
Composition:
Each ml contains:
Nandrolone Decanoate USP 250 mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Indications and usage:
Gaiadeca is indicated for the management of the anaemia of renal Insufficiency and has been shown to increase haemoglobin and red cell mass.
Drug Interactions:
Anticoagulants: Anabolic steroids may increase sensitivity to oral anticoagulants. Dosage of the anticoagulant may have to be decreased in order to maintain the prothrombin time at the desired therapeutic level. Patients receiving oral anticoagulant therapy require close monitoring, especially when anabolic steroids are started or stopped Anabolic steroids may decrease levels of thyroxine-binding globulin, resulting In decreased total 14 serum levels and increased resin uptake of T3 and 14. Free thyroid hormone levels remain unchanged.
Overdosage:
There have been no reports of acute overdosage with the anabolics.
Adverse reactions:
Hepatic: Choiestatic jaundice with, rarely, hepatic necrosis and death. Hepatocellular neothasms and peliosis hepatitis have been reported in association with long-term use of androgenic anabolic steroids, particularly those that are 17-alpha-alkylated.
In men: Prepubertal: Phallic enlargement and increased frequency of erections. Postpubertal: Inhibition of testicular function, testicular atrophy and otigospermia, impotence, chronic priapism, epididymitis and bladder irritability.
In women: Clitoral enlargement, menstrual irregularities.
In both sexes: Increased or decreased libido. Hormone levels remain unchanged.
CNS: Habituation, excitation, insomnia, depression.
Gastrointestinal: Nausea, vomiting, diarrhea.
Hematologic: Bleeding in patients on concomitant anticoagulant therapy (see Precaution, Drug Interactions). Breast Gynecomastia.
Larynx: Deepening of the vioce in women.
Hair: Hirsutism and male pattern baldness in women.
Skin: Acne (especially in women and prepubertal boys).
Skeletal: Premature closure of epiphyses children (see Precaution, Pediatric Use)
Fluid and electrolytes: Edema retention of serum electrolytes (sodium chloride, potassium phosphate, calcium).
Metabolic/endocrine: Decreased glucose tolerance, increased serum levels of low-density lipoproteins and decreased levels of high-density lipoproteins, increased creatine and creatinine excretion, increased serum levels of creatine phosphokinase. Some virilizing changes in women are irreversible even after prompt discontinuance of therapy and are not prevented by concomitant use of estrogens.
Precautions:
Gaiadeca should be used with caution in patients with any disease, especially cancer of the prostate or breast, liver, heart. Kidney disease, allergy, enlarged prostate. High dosage, long term use of androgens has been related to liver cancer. This drug should not be used during pregnancy or lactation. Females should be monitored for signs virilization, such as deepening of the voice. facial haft, acne, menstrual irregularity or clitoral enlargement, consult the doctor promptly if any of these symptoms occur. Use in children should not be recommended due to the possibility this drug may have undesirable effects related to the growth of the child. It should be used with extreme caution in geriatric men because they are at higher risk for developing enlarged prostates or prostate cancer when using this medication.
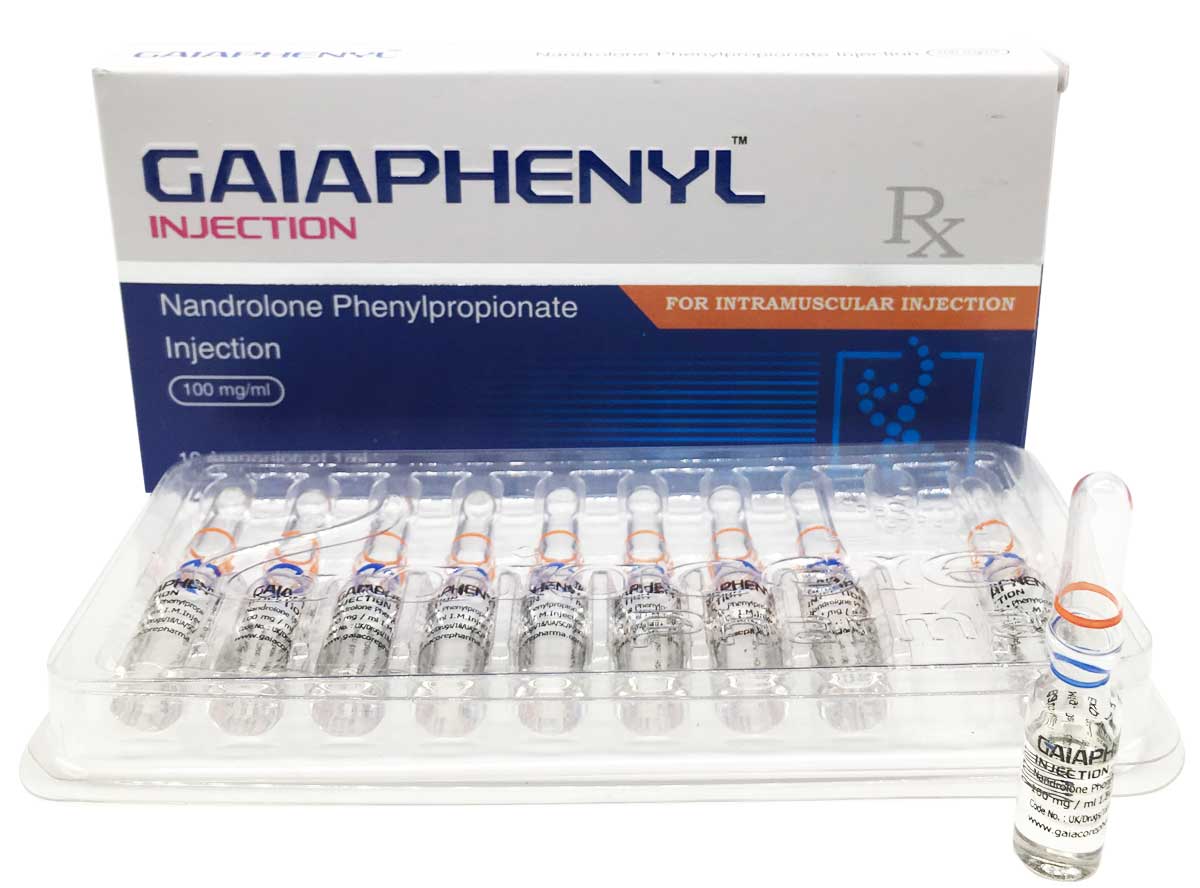
Gaiaphenyl
Composition:
Each ml contains:
Nandrolone Phenylphropionate 100mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
PHARMACOLOGICAL ACTION:
Gaiaphenyl is an injectable anabolic preparation. After injection, nandrolone phenylpropionate is gradually released from the intramuscular depot and subsequently hydrolysed into nandrolone.
INDICATIONS:
Certain cases of disseminated breast cancer in women. Osteoporosis due to androgen deficiency in hypogonadal males.
DOSAGE AND DIRECTIONS FOR USE:
GAIAPHENYL should be administered by deep intramuscular injection. Adult dose: 50-100 mg every 3 weeks.
SIDE-EFFECTS AND SPECIAL PRECAUTIONS:
Virilisation which appears in sensitive women as hoarseness, acne, hirsutism, and increased libido; in prepubertal boys as an increased frequency of erections and phallic enlargement, and in girls as an increase of pubic hair and clitoral hypertrophy. Hoarseness may be the first symptom of vocal change which may end in a long-lasting, sometimes irreversible deepening of the voice.
OTHER ADVERSE REACTIONS MAY INCLUDE:
Oligospermia and decreased ejaculatory volume;
Suppression of ovarian activity, atrophy of the breasts and endometrial tissue. Amenorrhoea and inhibition of spermatogenesis. Water and salt retention. Premature epiphyseal closure. If signs of virilisation develop, treatment should be discontinued. Increase in nitrogen retention and skeletal weight; Oedema; Increased vascularity of the skin; Increased growth of the bone; Elderly males may become over-stimulated.
PATIENTS WITH THE FOLLOWING CONDITIONS SHOULD BE MONITORED:
Latent or overt cardiac failure, renal dysfunction, hypertension, epilepsy or migraine (or a history of these conditions), since anabolic steroids may induce salt and fluid retention; diabetes, since anabolic steroids may improve the glucose tolerance and decrease the need for insulin or other antidiabetic drugs; incomplete statural growth, since anabolic steroids in high dosages may accelerate epiphyseal closure; skeletal metastases, since anabolic steroids may induce hypercalcaemia and hypercalciuria in these patients.
INTERACTIONS:
Liver-enzyme-inducing agents may reduce the effects of Gaiaphenyl by enhancing its metabolism in the liver.
Gaiadepot
Composition:
Each ml contains:
Testosterone Enanthate USP 250 mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Indications and usage :
Gaiadepot is indicated for replacement therapy in the male in conditions associated with symptoms of deficiency or absence of endogenous testosterone.
-
Primary hypogonadism (Congential or acquired) - testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, or orchidectomy.
-
Hypogonadotropic hypogonadisrn (congenital or acquired)-idiopathic gonadotropin or LHRH deficiency, or pituitary-hypothalamic injury from tumours, trauma, or radiation.
Contraindications:
-
Known hypersensitivity to the drug.
-
Males with carcinoma of the breast.
-
Males with known or suspected carcinoma of the prostate gland.
-
Women who are or who may become pregnant.
-
Patients with serious cardiac, hepatic or renal disease.
Drug Interaction:
Androgens may increase sensitivity to oral anticoagulants. Dosage of the anticoagulant may require reduction in order to maintain satisfactory therapeutic hypoprothrombinemia. Concurrent administration of oxyphentoutazone and androgens may result in elevated serum levels of oxyphenbutazone. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and therefore, insulin requirements.
Overdosage:
There have been no reports of acute overdosage with the androgens.
Precautions:
General: Patients with benign prostatic hypertrophy may develop acute urethral obstruction. Priapism or excessive sexual stimulation may develop. Oligosperrnia may occur after prolonged administration or excessive dosage. If any of these effects appear, the androgen should be stopped and if restarted, a lower dosage should be utilized. GAIADEPOT should not be used interchangeably with testosterone propionate because of differences in duration of action. Gaiadepot is not for intravenous use.
Information for patients:
Patients should be instructed to report any of the following nausea. vomiting, changes in skin color, ankle swelling, too frequent or persistent erections of the penis.
Laboratory tests:
Hemoglobin and hernatocrit levels (to detect polycytbernia) should be checked periodically in patients receiving long-term androgen administration. Serum cholesterol may increase during androgen therapy. Drug/Laboratory test Interferences: Androgens may decrease levels of thyroxine-binding globulin, resulting in decreased total T4 serum levels and increased resin uptake of T3 and T4. Free thyroid hormone levels remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
Careinogenesis:
There are rare reports of hepatocellular carcinoma in patients receiving long term therapy with androgens in high doses. Withdrawal of the drugs did not lead to regression of the tumours in all cases. Geriatric patients treated with androgens may be at an increased risk of developing prostatic hypertrophy and prostatic carcinoma although conclusive evidence to support this concept is lacking.
Pregnancy: Teratogenic Effects. Pregnancy Category X. Nursing mother: GAIADEPOT is not recommended for use in nursing mothers.
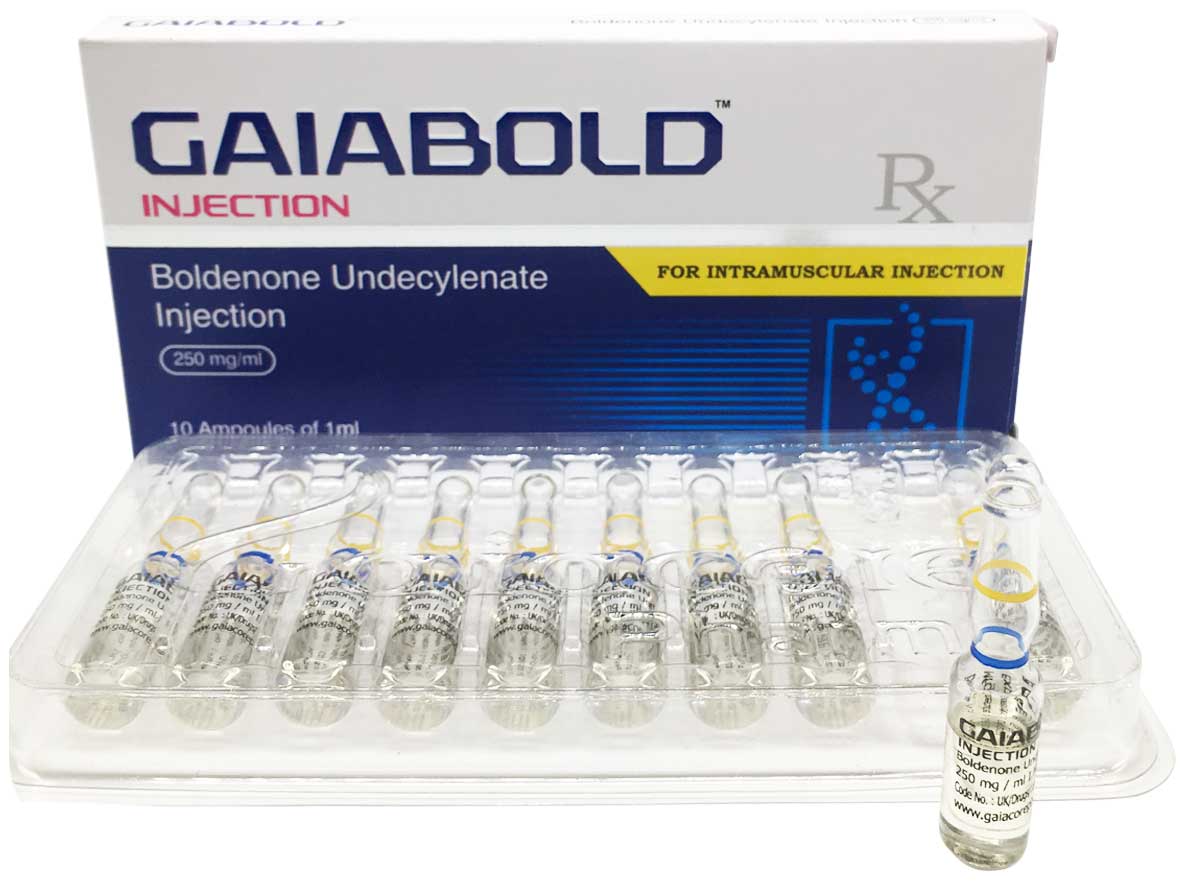
Gaiabold
Composition:
Each ml contains:
Boldenone Undecylenate 250 mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Indications and usage:
Gaiabold is indicated for replacement therapy in the male in conditions associated with symptoms of deficiency or absence of endogenous testosterone.
1.Primary hypogonadisrn (congential or acquired-testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, or orchidectomy.)
-
Hypogonadotropic hypogonadism (congenital or acquired)-idiopathic gonadotropin or LHRH deficiency, or pituitary-hypothalamic injury from tumours, trauma, or radiation.
Contraindications:
-
Known hypersensitivity to the drug.
-
Males with carcinoma of the breast.
-
Males with known or suspected carcinoma of the prostate gland.
-
Women who are or who may become pregnant.
-
Patients with serious cardiac, hepatic or renal disease.
Drug Interactions:
Androgens may increase sensitivity to oral anticoagulants. Dosage of the anticoagulant may require reduction in order to maintain satisfactory therapeutic hypoprothrombinemia. Concurrent administration of oxyphenbutazone and androgens may result in elevated serum levels of oxyphenbutazone. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and therefore insulin requirements.
Overdosage:
There have been no reports of acute overdosage with the androgens.
Precautions:
General: Patients with benign prostatic hypertrophy may develop acute urethral obstruction. Priapism or excessive sexual stimulation may develop. Oligospermia may occur after prolonged administration or excessive dosage. If any of these effects appear, the androgen should be stopped and if restarted, a lower dosage should be utilized. GAIABOLD should not be used interchangeably with testosterone propionate because of differences in duration of action. Gaiabold is not for intravenous use.
Information for patients: Patients should be instructed to report any of the following nausea, vomiting. changes in skin color, ankle swelling, too frequent or persistent erections of the penis.
Laboratory tests: Hemoglobin and hematocrit levels (to detect polycythemia) should be checked periodically in patients receiving long-term androgen -administration. Serum cholesterol may increase during androgen therapy. Drug/Laboratory test
Interferences: Androgens may decrease levels of thyroxine-binding globulin, resuhng in decreased total T4 serum levels and increased resin uptake of T3 and T4. Free thyroid hormone levels remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
Carcinogenesis : There are rare reports of hepatocellular carcinoma in patients receiving long term therapy with androgens in high doses. Withdrawal of the drugs did not lead to regression of the tumours in all cases. Geriatric patients treated with androgens may be at an increased risk of developing prostatic hypertrophy and prostatic carcinoma although conclusive evidence to support this concept is lacking.
Nursing mother: Gaiabold is not recommended for use in nursing mothers.
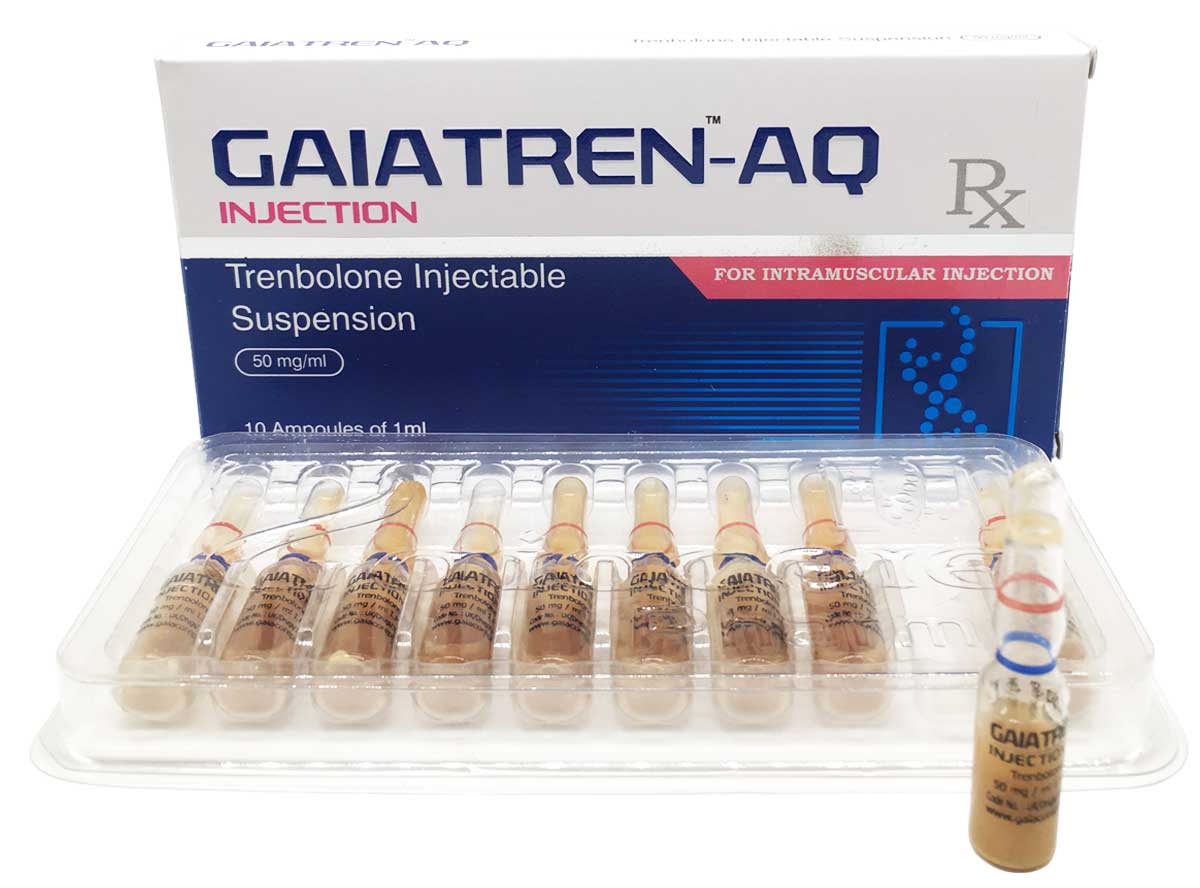
Gaiatren-AQ
Composition:
Each ml contains:
Trenbolone Base 50mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. For more information check the website Laboratory Tested Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
PHARMACOLOGICAL ACTION:
Gaiatren-AQ is an injectable anabolic preparation. After injection, trenbolone suspension is gradually released from the intramuscular depot and subsequently hydrolysed into nandrolone.
INDICATIONS:
Fast acting anabolic steroid with strong androgenic and anabolic activity. For increased rate of weight gain and improved food efficiency.
DOSAGE AND DIRECTIONS FOR USE:
2-3 times weekly 50-100 mg, during prolonged usage.
SIDE-EFFECTS AND SPECIAL PRECAUTIONS:
Prolonged usage or overdosage may cause androgenic related side-effect like hepatotoxicity, aggressively.
PATIENTS WITH THE FOLLOWING CONDITIONS SHOULD BE MONITORED:
Latent or overt cardiac failure, renal dysfunction, hypertension, epilepsy or migraine (or a history of these conditions), since anabolic steroids may induce salt and fluid retention; diabetes, since anabolic steroids may improve the glucose tolerance and decrease the need for insulin or other antidiabetic drugs; incomplete statural growth, since anabolic steroids in high dosages may accelerate epiphyseal closure; skeletal metastases, since anabolic steroids may induce hypercalcaemia and hypercalciuria in these patients.
INTERACTIONS:
Liver-enzyme-inducing agents may reduce the effects of Gaiatren-AQ by enhancing its metabolism in the liver.
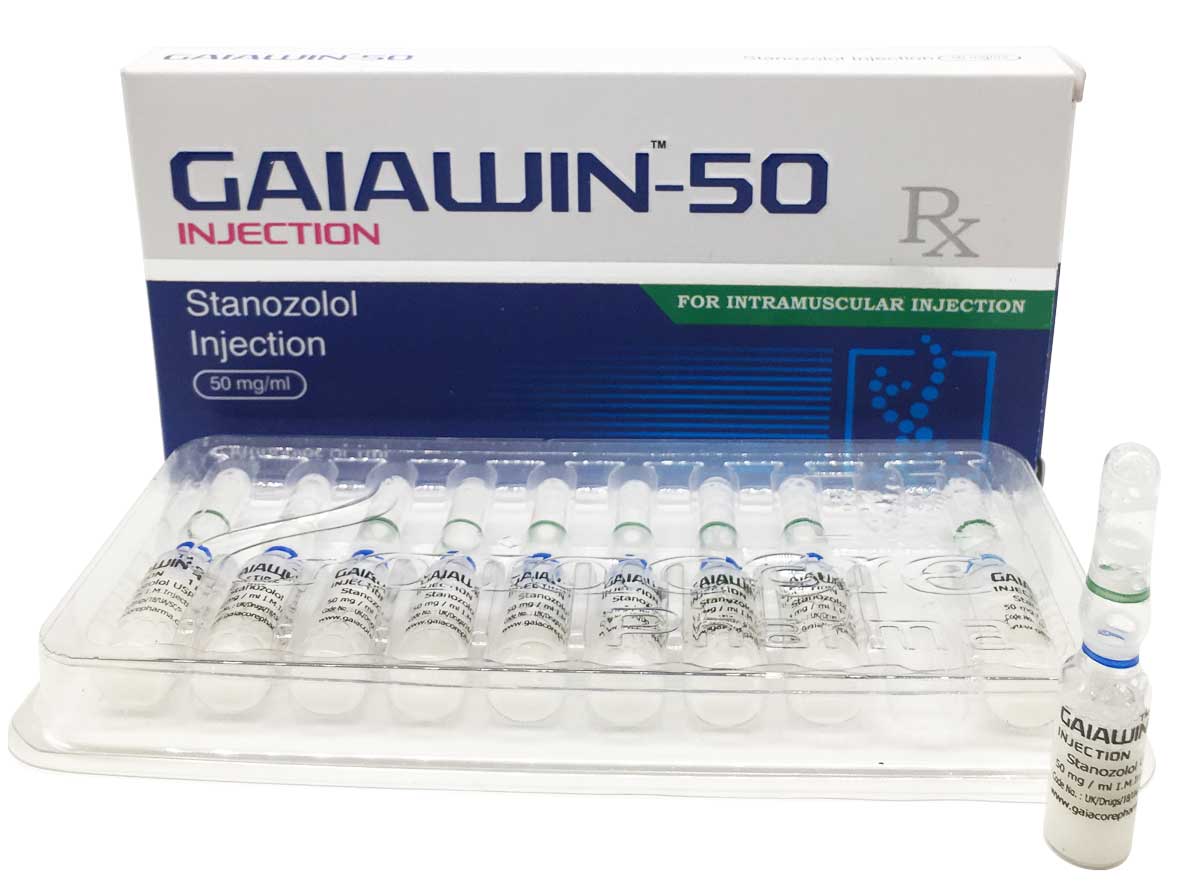
Gaiawin-50
Composition:
Each ml contains:
Stanozolol USP 50 mg
Water For Injection USP q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Indications and usage
Hereditary angioedema : Gaiawin-50 is indicated prophylactically to decrease the frequency and severity of attacks of angioedema.
Drug interactions:
Anabolic steroids may increase sensitivity to anticoagulants therefore, dosage of an anticoagulant may have to be decreased in order to maintain the prothrombin time at the desired therapeutic level.
Over dosage:
There have been no reports of acute over dosage with the androgens.
Precautions:
General: women should be observed for signs of virilisation (deepening of the voice, hirsutism, acne, and clitoromegaly). To prevent irreversible change, drug therapy must be discontinued, or the dosage significantly reduced. Such virilisation is usual following androgenic anabolic steroid use at high dosages. some virilising changes in women are irreversible even after prompt discontinuance of therapy and are not prevented by concorntant use of estrogens. Menstrual irregularities may also occur. The insulin or oral hypoglycemic dosage may need adjustment in diabetic patients who receive anabolic steroids.
Information for the Patient: The physician should instruct patients to report any of the following side effects of androgens.
Adult or Adolescent Males: Too frequent or persistent erections of the penis, appearance of aggravation of acne.
Women: Hoarseness, acne changes in menstrual periods, or more hair on the face.
All Patients: Any nausea, vomiting, changes in skin colour, or ankle swelling.
Gaiatren
Composition:
Each ml contains:
Trenbolone Acetate USP 100mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
PHARMACOLOGICAL CLASSIFICATION:
Anabolic Steroids
PHARMACOLOGICAL ACTION:
Trenbolone Acetate is a man - made steroid, similar to the naturally occurring steroid, testosterone. Trenbolone Acetate is used to promote weight gain following extensive surgery, chronic infection, or severe trauma, and in other cases that result in inadequate weight gain or maintenance. Trenbolone Acetate is also used to decrease muscle loss caused by treatment with corticosteroids and to reduce bone pain associated with osteoporosis. Anabolic steroids bind to specific receptors present especially in reproductive tissue, muscle and fat. The anabolic steroids reduce nitrogen excretion from tissue breakdown in androgen deficient men. They are also responsible for normal male sexual differentiation. The ratio of anabolic ("body-building") effects to androgenic (Virilizing) effects may differ among the members of the class, but in practice all agents posses both properties to some degree. There is no clear evidence that anabolic steroids enhance overall athletic performance.
DOSAGE AND ADMINISTRATION:
2-3 times weekly 50-200mg , during prolonged usage.
SIDE EFFECTS:
It is very toxic to the kidneys, and will cause acne and aggression, and therefore not recommended to be used by rookies in the world of steroids. Acne; enlarging penis; increased frequency of erection; acne or oily skin; enlarging clitoris; deepening of voice; irregular menstrual periods; unnatural hair growth.
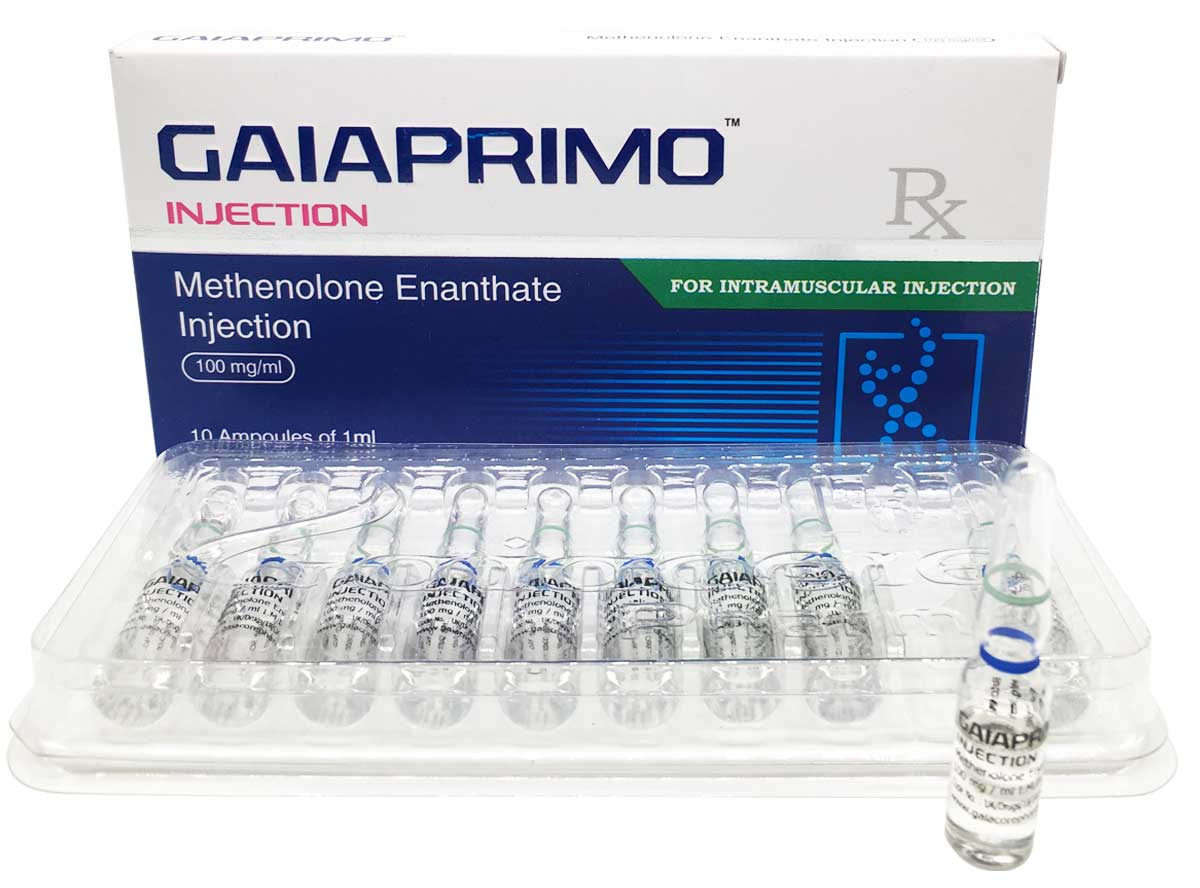
Gaiaprimo
Composition:
Each ml contains:
Methenolone Enanthate 100mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
PHARMACOLOGICAL ACTION:
GAIAPRIMO is an injectable anabolic preparation. After injection, methenolone enanthate is gradually released from the intramuscular depot and subsequently hydrolysed into nandrolone.
INDICATIONS:
Gaiaprimo should be used with caution in patients with any disease, especially cancer of the prostate or breast, liver, heart. Kidney disease, allergy, enlarged prostate. High dosage, long term use of androgens has been related to liver cancer. This drug should not be used during pregnancy or lactation. Females should be monitored for signs virilization, such as deepening of the voice. facial haft, acne, menstrual irregularity or clitoral enlargement, consult the doctor promptly if any of these symptoms occur. Use in children should not be recommended due to the possibility this drug may have undesirable effects related to the growth of the child. It should be used with extreme caution in geriatric men because they are at higher risk for developing enlarged prostates or prostate cancer when using this medication.
DOSAGE AND DIRECTIONS FOR USE:
GAIAPRIMO should be administered by deep intramuscular injection. Adult dose: 50-100 mg every 3 weeks.
SIDE-EFFECTS AND SPECIAL PRECAUTIONS:
Virilisation which appears in sensitive women as hoarseness, acne, hirsutism, and increased libido; in prepubertal boys as an increased frequency of erections and phallic enlargement, and in girls as an increase of pubic hair and clitoral hypertrophy. Hoarseness may be the first symptom of vocal change which may end in a long-lasting, sometimes irreversible deepening of the voice.
OTHER ADVERSE REACTIONS MAY INCLUDE:
Oligospermia and decreased ejaculatory volume; Suppression of ovarian activity, atrophy of the breasts and endometrial tissue. Amenorrhoea and inhibition of spermatogenesis. Water and salt retention. Premature epiphyseal closure. If signs of virilisation develop, treatment should be discontinued. Increase in nitrogen retention and skeletal weight; Oedema; Increased vascularity of the skin; Increased growth of the bone; Elderly males may become over-stimulated.
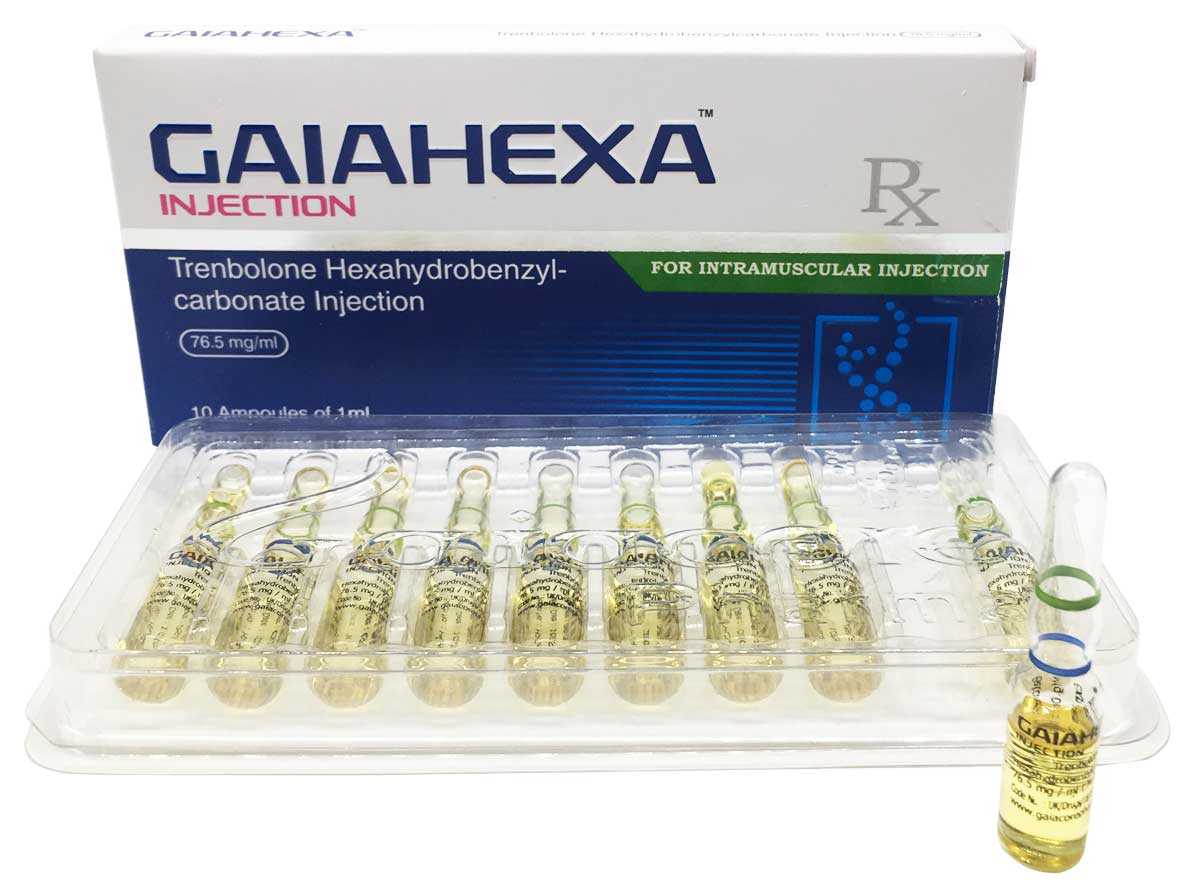
Gaiahexa
Composition:
Each ml contains:
Trenbolone Hexahydrobenzylcarbonate 76.5 mg
Oil Base q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
PHARMACOLOGICAL CLASSIFICATION:
Mechanism of Action
It has both anabolic and androgenic effects. Once metabolized, the drugs have the effect of increasing ammonium ion uptake by muscles, leading to an increase in the rate of protein synthesis. It may also have the secondary effects of stimulating appetite and decreasing the rate of catabolism, as all anabolic steroids are believed to; however, catabolism likely increases significantly once the steroid is no longer taken. At least one study in rats has shown trenbolone to cause gene expression of the androgen receptor at least as potent as DHT. This evidence tends to indicate trenbolone can cause an increase in male secondary sex characteristics without the need to convert to dihydrotestosterone.
Pharmacokinetics
To increase its effective half-life, trenbolone is administered as a prodrug as an ester conjugate such as trenbolone acetate, trenbolone enanthate, or trenbolone hexahydrobenzylcarbonate . Plasma lipases then cleave the ester group in the bloodstream leaving free trenbolone. Trenbolone compounds have a binding affinity for the androgen receptor five times as high as that of testosterone. Trenbolone also binds with high affinity to the progesterone receptor, Trenbolone binds to the glucocorticoid receptor as well.
Pharmacodynamics
Studies on metabolism are mixed, with some studies showing that it is metabolized by aromatase or 5α-reductase into estrogenic compounds such as estradiol, or into dihydrotestosterone. Trenbolone and 17epi-trenbolone are both excreted in urine as conjugates that can be hydrolyzed with beta-glucuronidase. This implies that trenbolone leaves the body as beta-glucuronides or sulfates.
INDICATIONS:
Trenbolone hexahydrobenzylcarbonate is a slow-acting injectable ester of the potent anabolic steroid trenbolone.Trenbolone appears most commonly as trenbolone acetate, which is a much faster-acting form of the drug The hexahydrobenzylcarbonate ester used here extends the release of trenbolone for more than 2 weeks, which has always been thought of as more suitable for human use due to the less frequent injection schedule. The base steroid trenbolone is roughly three times more androgenic than testosterone, making it a fairly potent androgen. It also displays about 3 times greater tissue-building activity in comparison to its androgenic properties, making its official classification as that of an anabolic steroid. The muscle building effect of trenbolone is often compared to such popular bulking agents as testosterone, but without the same estrogen related side effects. It is most commonly identified as a lean-mass-building drug, and is extremely popular with athletes for its ability to promote the rapid buildup of strength, muscle size, and definition.
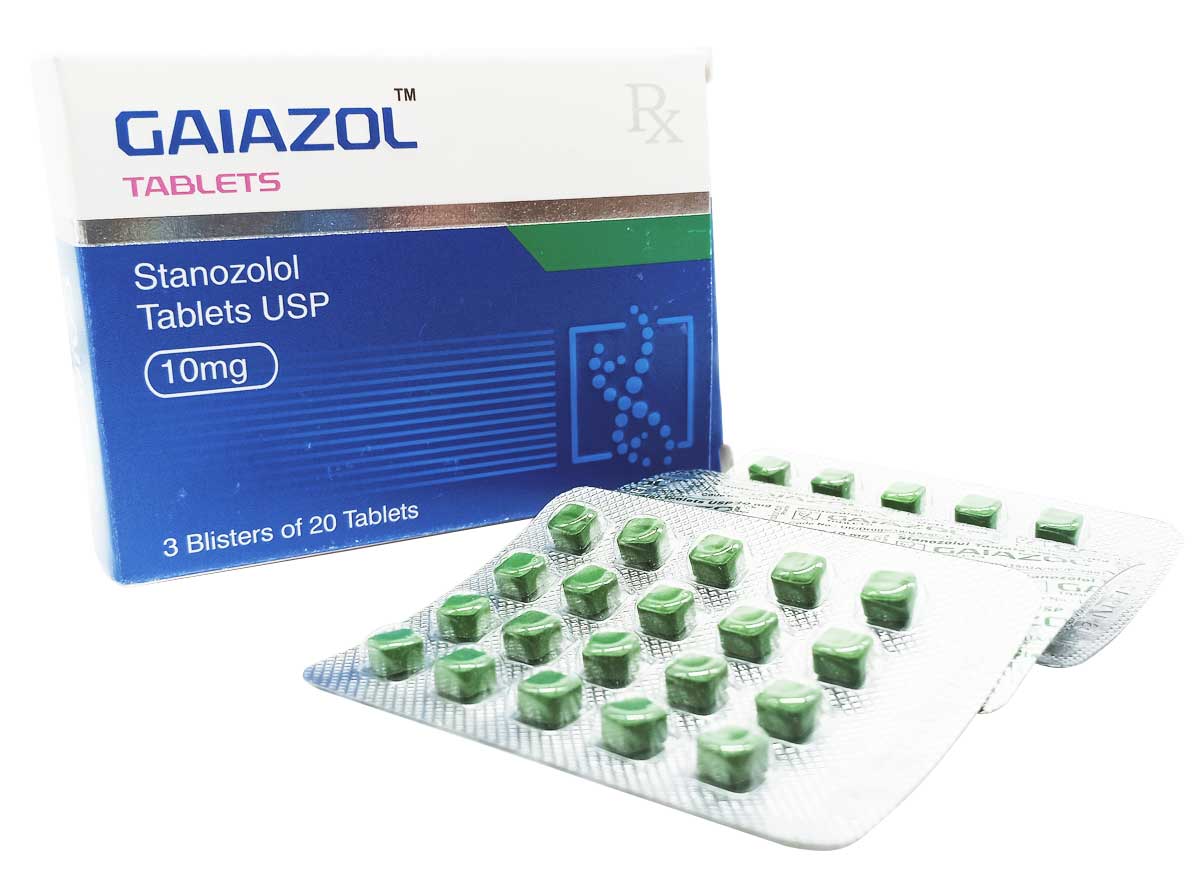
Gaiazol
Composition:
Each tablet contains:
Stanozolol USP 10mg
Excipients q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Indications and usage:
Hereditary Angioedema: for prophylactic use to decrease frequency and severity of attacks of angioedema. Muscle Anabolism: for adjunctive therapy in patients for weight gain following severe muscular atrophy associatedwith extensive surgery, chronic infections, long term hospitalization, or sever trauma. Corticosteroid atrophy: to reduce muscle wasting during prolonged corticosteroid use.
Drug interactions:
Gaiazol may increase sensitivity to anticoagulants; therefore, dosage of an anticoagulant may have to be decreased in order to maintain the prothrombin time at the desired therapeutic level.
Overdosage :
Cholestatic hepatitis and jaundice occur with 17 alpha alkylated androgens at relatively low doses. If cholestic hepatitis with jaundice appears the stanozolol dose should be discontinued. If liver function tests become abnormal, the patient should be monitored closely and the aetiology determined. In patient with breast cancer anabolic steroid may cause hypercalcemia by stimulating osteolysis in this case the drug should be discontinued. Edema with or without congestive heart failure may be a serious complication in patients with pre existing cardiac, renal, or hepatic disease.
Precautions:
General: Women should be observed for signs of virilisation (deepening of the voice, hirsutism, acne, and clitoromegaly). To prevent irreversible change, drug therapy must be discontinued, or the dosage significantly reduced. Such virilisation is usual following androgenic anabolic steroid use at high doses. Some virilising changes in women are irreversible even after prompt discontinuance of therapy and are not prevented by concomitant use of estrogens. Menstrual irregularities may also occur. The insulin or oral hypoglycemic dosage may need adjustment in diabetic patients who receive anabolic steroids.
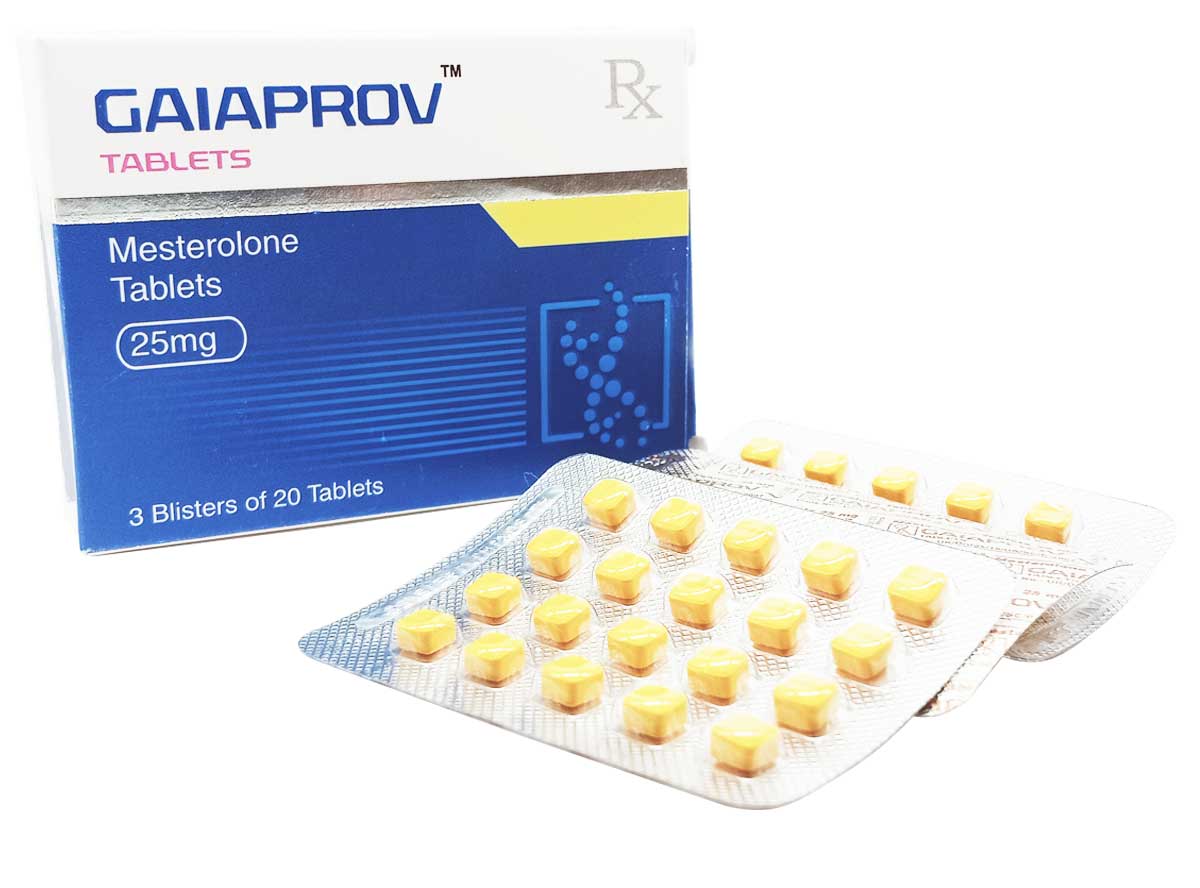
Gaiaprov
Composition:
Each tablet contains:
Mesterolone 25mg
Excipients q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
PHARMACOLOGICALCLASSIFICATION
Male sex hormones.
PHARMACOLOGICAL ACTION
Mesterolone balances a deficiency of androgen formation which begins to fall gradually with increasing age. Therefore, Mesterolone is suitable for treatment of all conditions caused by deficient endogenous androgen formation. In the recommended therapeutic dosage, Mesterolone will not impair spermatogenesis. Mesterolone is especially well tolerated by the liver.
INDICATIONS
• Declining physical activity and mental alertness in middle- and old-aged men Reduced efficiency, easy fatigability, lack of concentration, weak memory, disturbances of libido and potency, irritability, disturbances of sleep, depressive moods, and general vegetative complaints are often attributed to androgen-deficiency. These complaints can be overcome or improved by the use of Mesterolone tablets.
• Potency disturbances
Mesterolone overcomes potency disturbances due to androgen-deficiency. It may also be of use as supplementary therapy in cases of diminished potency where androgen-deficiency is not the primary cause.
• Hypogonadism
Growth, development and function of androgen-dependent target organs are stimulated by Mesterolone. It promotes development of secondary male sex characteristics in cases of prepuberal hypogonadism. Full clinical and laboratory investigations are necessary in all cases of young patients prior to commencement of treatment. Mesterolone tablets may also be used as a substitution therapy in cases where a loss of gonadal function has occurred post-puberally.
• Infertility
Oligozoospermia and deficient Leydig-cell secretion may be the cause of infertility. With Mesterolone treatment, sperm count can be increased, the quality improved and, furthermore, a higher fructose concentration up to normal values can be achieved thus increasing the chances of procreation.
DOSAGE AND DIRECTIONS FOR USE
If not otherwise directed by the physician, the following dosage is recommended: In declining physical activity and potency disturbances Commencement of treatment: 1 Mesterolone tablet of 25 mg three times daily. Continuation of treatment: 1 Mesterolone tablet of 25 mg twice or once daily. According to type and severity of the complaints, a course of Mesterolone lasting four to six weeks or a prolonged uninterrupted treatment over several months is recommended. If required, the course of treatment may be repeated several times.
Hypogonadism requires continuous therapy
For development of secondary male sex characteristics 1 Mesterolone tablet of 25 mg 3-4 times daily for several months. As maintenance dose 1 Mesterolone tablet of 25 mg twice or three times daily will be sufficient.
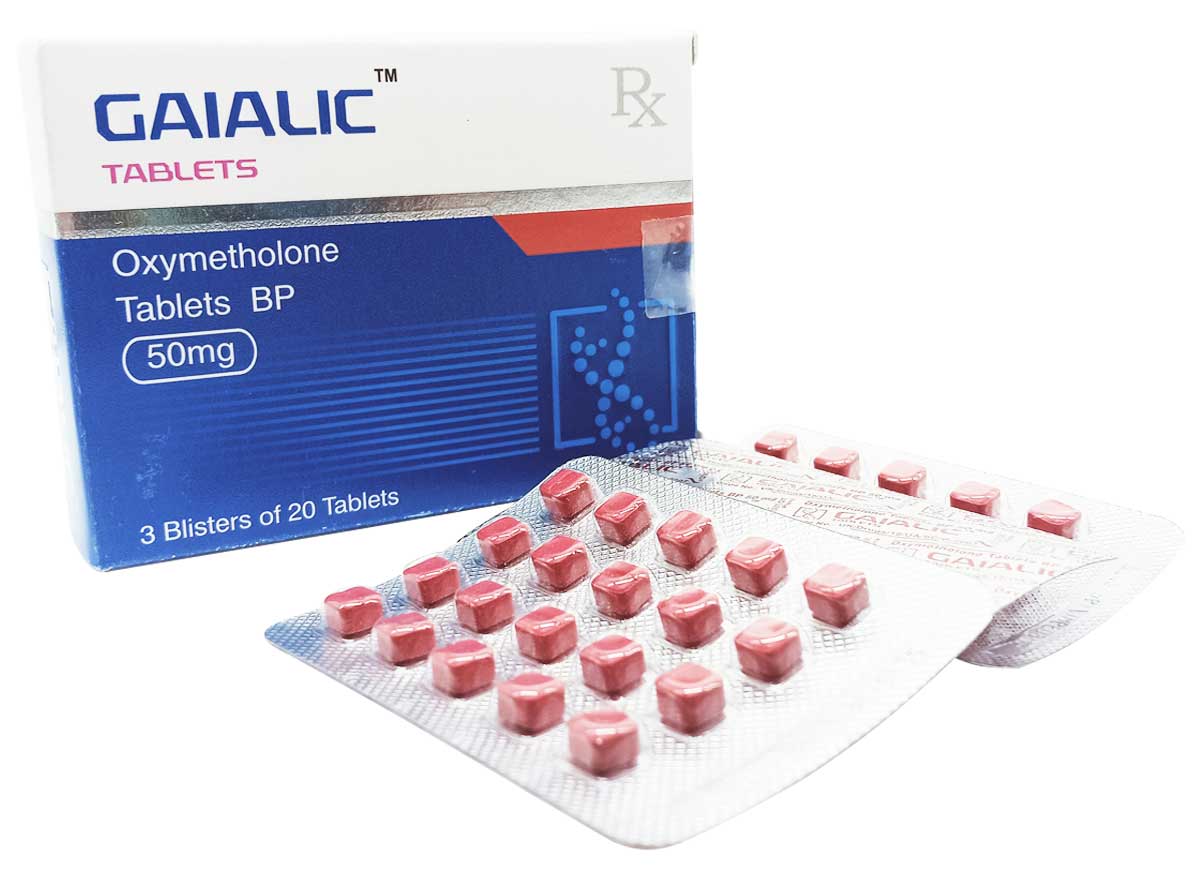
Gaialic
Composition:
Each tablet contains:
Oxymetholone BP 50mg
Excipients q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Indications and usage:
Rapid restore muscle tissue atrophied during recovery from a traumatic injury. Offset muscle catabolism in patients with a wasting syndrome. Treat certain types of anemia which are non-responsive to first line agents.
Drug interactions:
Oral hypoglycaemic agents: may inhibit the metabolism of oral hypoglycaemic agents which may equire adjustments of dosage. Anti coagulants: Patients on anticoagulants should be carefully monitored during anabolic steroid therapy as anabolic steroids may increase sensitivity to oral anticoagulants. Patients should be monitored regularly during anabolic steroid therapy, particularly during initiation and termination of therapy.
Over dosage:
Signs and symptoms of over dosage are those associated with the known effects of the drug. See adverse reactions section. Treatment is symptomatic and supportive. Evacuate stomach contents by emesis and, if indicated, lavage, taking care to prevent aspiration. Monitoring of liver function is advised.
Precautions:
Elevated liver enzymes and in extreme cases hepatic liver dysfunction may occur, 17 alpha alkylated androgens may cause cholestatic hepatitis and jaundice, particularly with larger dosages or prolonged treatment. Liver function should be monitored for changes including serum bilrubin, aspartete aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatise (AP). Edema may be increased in patients on concurrent adrenal cortical steroid or ACTH therapy. Anabolic steroid hormones may increase low density lipoproteins (LDL) and decrease high density lipoproteins (HDL). Lipids levels generally return to normal upon discontinuation of treatment.
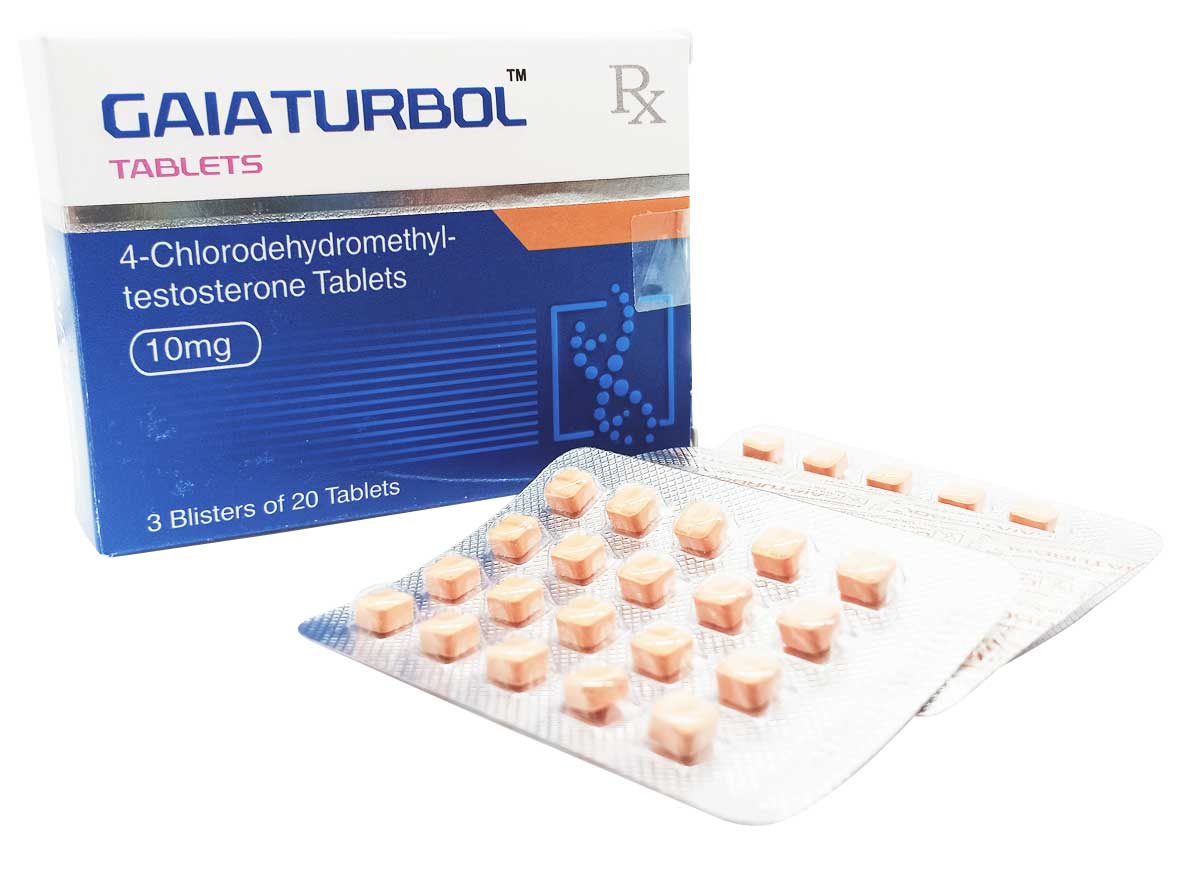
Gaiaturbol
Composition:
Each tablet contains:
4-Chlorodehydromethyl-testosterone Tablets 10mg
Excipients q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. For more information check the website Laboratory Tested Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
PHARMACOLOGICALACTION
GAIATURBOL is considered to be an oral anabolic steroid mainly used for androgynous reduction. For this reason, androgynous activity is claimed to be 6%, while anabloic one is 53%. Chlorodehydromethyl Testosterone, together with other anabolic and androgynous steroids, penetrates into the cell membrane and cytoplasmic receptors. When interacting they reach the nucleus where Chlorodehydromethyl Testosterone affects certain nuclear receptors. The active substance combines with the DNA and
performs as a transcription factor that causes the synthesis of specific RNA and proteins. Chlorodehydromethyl Testosterone has mainly an anabolic effect which is mostly noticeable in the striated muscles and bones, together with other tissues with the intensive area (bone marrow, mucous membrane, etc). Its effect primarily determines the rate of muscle growth and mass increase. An essential contribution to the realization of metabolic diseases is considered to be the anabolic affect: positive nitrogen balance
(nitrogen retention).
PHARMACOKINETIC PROPERTIES:
The metabolism of Chlorodehydromethyl Testosterone in the body takes from 6 to 8 days
INDICATIONS:
Protein synthesis disorder, Traumas , Burns, Postoperative Infections, kidney failure, Osteoporosis, Corticosteroid therapy, Aplastic anemia, Negative nitrogen balance, Muscular atrophy when the patients have spin, Anorexia, Loss of appetite, Weight loss, Leukemia
DOSAGE:
The therapy with anabolic steroids is complementary and we can't replace the primary treatment. The treatment depends from the patients and the side effects.
Adults: the steroid effect is varied. The dose for adults is 20-50 mg for a day (2-6 weeks)
DRUG INTERACTIONS
When indirect anticoagulants are used together with anabolic steroids, their concentration and effects are reduced, so the dose needs to be reduced. It is necessary to monitor the prothrombin time. Concomitant use of ACTH and corticosteroids in patients with edema may worsen but the likelihood is very small. The free fraction of thyroid hormone levels remains unchanged.
Gaiadrolone
Composition:
Each tablet contains:
Oxandrolone USP 10mg
Excipients q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
CONTRA-INDICATIONS:
Not intended for use in children. Known or suspected prostatic carcinoma and mammary carcinoma in the male. Not intended for use in female patients other than those with disseminated breast cancer. Contraindicated in nephrosis or the nephrotic phase of nephritis, cardiac and renal failure, hypercalcaemia, oedema, jaundice, liver disease with impaired bilirubin excretion, testicular and hepatic carcinoma.
DOSAGE AND DIRECTIONS FOR USE: GAIADROLONE should be taken orally per day in divided doses. Adult dose: 5-10 mg every day.
SIDE-EFFECTS AND SPECIAL PRECAUTIONS:
Virilisation which appears in sensitive women as hoarseness, acne, hirsutism, and increased libido; in prepubertal boys as an increased frequency of erections and phallic enlargement, and in girls as an increase of pubic hair and clitoral hypertrophy. Hoarseness may be the first symptom of vocal change which may end in a long-lasting, sometimes irreversible deepening of the voice.
Over dosage:
Signs and symptoms of over dosage are those associated with the known effects of the drug. See adverse reactions section. Treatment is symptomatic and supportive. Evacuate stomach contents by emesis and, if indicated, lavage, taking care to prevent aspiration. Monitoring of liver function is advised.
Gaiabithiro
Each tablet contains:
Levothyroxine ( T4 ) 50 mcg
Liothyronine ( T3 ) 12.5 mcg
Excipients q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
PHARMACOLOGICAL ACTION: Thyroxine and Liothyronine have qualitatively similar biological actions, but Liothyronine (L-triiodothyronine) acts more promptly with a more intense but shorter lasting effect. The thyroid gland secretes both hormones and both are probably involved in maintaining the normal euthyroid state. Administration of thyroxine and Liothyronine provides a more natural replacement therapy than is given by one hormone alone. The soluble sodium salts are more efficiently and consistently absorbed than the free substances. Thyroid hormone drugs are natural or synthetic preparations containing T4 or T3 or both. T4 and T3 are produced in the human. thyroid gland by the iodination and coupling of the amino acid tyrosine. GAIABITHIRO is a synthetic preparation of T4 and T3 ina 4:1 weight-based ratio. These hormones enhance oxygen consumption by most tissues of the body and increase the basal metabolic rate and the metabolism of carbohydrates, lipids and proteins. Thus, they exert a profound influence on every organ system in the body and are of particular importance in the development of the central nervous system.
Mechanism of action:
The hormones, T4 and T3, are tyrosine-based hormones produced by the thyroid gland. Iodine is an important component in sis. The major secreted form of thyroid hormone is T4. T4 is converted T3, the more active thyroid hormone, by deiodinases in peripheral tissues. T3 acts in the body to increase basal metabolic rate alter protein synthesis and increase the body's sensitivity to catecholamines (such as adrenaline). Thyroid hormones are essential for proper development and differentiation of all cells of the human body. T4 and T3 regulate protein, fat and carbohydrate metabolism to varying extents. The most pronounced effect of the hormones is in altering how human cells use energetic compounds. The thyroid hormone derivatives bind to the thyroid hormone receptors initially to initiate their downstream effects.
INDICATIONS:
Gaiabithiro Tablets are indicated:
- As replacement or supplemental therapy in patients with hypothyroidism of any etiology, except transient hypothyroidism during the recovery phase of subacute thyroiditis. This category includes cretinism, myxedema, and ordinary hypothyroidism in patients of any age (children, adults, the elderly), or state (including pregnancy); primary hypothyroidism resulting from functional deficiency, primary atrophy, partial or total absence of thyroid gland, or the effects of surgery, radiation, or drugs, with or without the presence of goiter; and secondary (pituitary), or tertiary (hypothalamic) hypothyroidism.
- As pituitary TSH suppressants, in the treatment or prevention of various types of euthyroid goiters, including thyroid nodules, sub-acute or chronic lymphocytic
Gaiabol
Composition:
Each tablet contains:
Methandrostenolone 10mg
Excipients q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Pharmacological action:
Has significant anabolic activity (increases protein synthesis) at a relatively low androgenic acting (similar to the action of male sex hormones). This effect is manifest in a positive nitrogen balance in the body and improves general state of person. The balance of calcium also has a positive effect: Methandrostenolone promotes the flow of calcium into bone tissue. Methandrostenolone is indicated in all diseases and conditions, in which is shown anabolic effect (accelerating growth of protein) and fortifying effect.
Indications of Methandrostenolone:
- Violation of protein metabolism-asthenia (weakness)
- Cachexia (extreme exhaustion) of different origin, after severe trauma, surgery, burns, infections, and other diseases associated with protein loss (infections, burns, radiationtherapy, prolonged use of corticosteroids, etc.)
- Osteoporosis (malnutrition bone, accompanied by an increase in its fragility)
- Inpediatric (child) practice
- Growth retardation
- Anorexia (lack of appetite)
- Depression nutrition etc.
Gaiaterol
Each tablet contains:
Clenbuterol HCI BP 40mcg
Excipients q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Clinical Pharmacology:
Clenbuterol exerts a pronounced action on the metabolism; with both anabolic and anti-catabolic effects. Clenbuterol has been demonstrated to increase the rate of protein synthesis in muscle tissue and to increase protein to fat ratio. Clenbuterol is a beta-2 adrenergic agonist relaxing airway muscle tissue and proving bronchodialation. Clenbuterol increases aerobic capacity, pulse perspiration and blood pressure; increases basal metabolic rate and temperature. Clenbuterol has an oral bioavailability of 89 to 98 % with a half- life of 36 to 48 hours after oral use.
Indication:
To increase the rate of protein synthesis in muscle tissue and to increase protein to fat ratio in human. Contraindication:
Children or geriatric patients Individuals who have been diagnosed of cardio-vascular problems should not take Clenbuterol. Women who are pregnant or may become pregnant Concomitantly with other CNS stimulants, adrenergic, or hypertensive agents In patients with hypersensitivity to Clenbuterol or other ingredients In patients with a history of GI bleeding, ulceration of the stomach, or gastritis
Drug Interaction:
Clenbuterol may enhance the action and side effects of other beta —adrenergic mimetics, theophylline and anti- cholinergic. Beta blockers may inhibit the action of Clenbuterol; thus a risk of bronchospasm exists with concomitant uses. Clenbuterol may alter the action of hypoglycemic agents, insulin sensitizers, insulin and insulin secretagogues. Adjust of dosage may be required. Monitor for loss of glycaemic control. There is an increased risk of arrhythmia with concomitant administration of halogenated carbohydrates for nacrosis; e.g. during surgery. Concomitant use with diuretics and digitalis glycosides requires periodic monitoring of serum electrolytes.
Concomitant use with other CNS stimulants should be avoided given the cumulative stimulatory effects and increased risk of adverse events. Concomitant use with MAO- inhibitors and tri- cyclic antidepressant may produce cardiac dysrhythmias. Adverse Reactions: CNS: Nervousness, tremor, ataxia, dizziness, restlessness, agitation, anxiety, insomnia and headache. Cardiovascular: palpitations, tachycardia, hypertension, angina, ventricular extra systole, myocardial infarction.
Gaiahalo
Composition:
Each tablet contains:
Fluoxymesterone USP 5mg
Excipients q.s
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Dosage and directions for use:
Muscle anabolism: 10 - 30 mg taken orally each day in divided doses for duration of 4-6 weeks.
Hereditary angioedema: The dosage requirement for continuous treatment of hereditary angioedema with Gaiahalo should be individualized on the basis of the clinical response of the patient. Commonly 2-6 mg taken orally each day in divided doses initially and reduced by physician thereafter.
Effective dose for male: 5 - 20 mg per day
Effective dose for female: 10 - 40 mg per day
Interaction:
Gaiahalo may increase sensitivity to anticoagulants; therefore, dosage of an anticoagulant may have to be decreased in order to maintain the prothrombin time at the desired therapeutic level.
Gaiatrenan
COMPOSITION:
Each ml contains:
Trenbolone Enanthate 200 mg
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Pharmacological Action:
Fast acting anabolic steroid for human with strong androgenic and anabolic activity. For increased rate of weight gain and improved feed efficiency.
Indications:
Certain cases of disseminated breast cancer in women. Osteoporosis due to androgen deficiency in hypogonadal males.
Contra-Indications:
Not intended for use in children.
Known or suspected prostatic carcinoma and mammary carcinoma in the male.
Not intended for use in female patients other than those with disseminated breast cancer.
Contraindicated in nephrosis or the nephrotic phase of nephritis, cardiac and renal failure, hypercalcaemia, oedema, jaundice, liver disease with impaired bilirubin excretion, testicular and hepatic carcinoma.
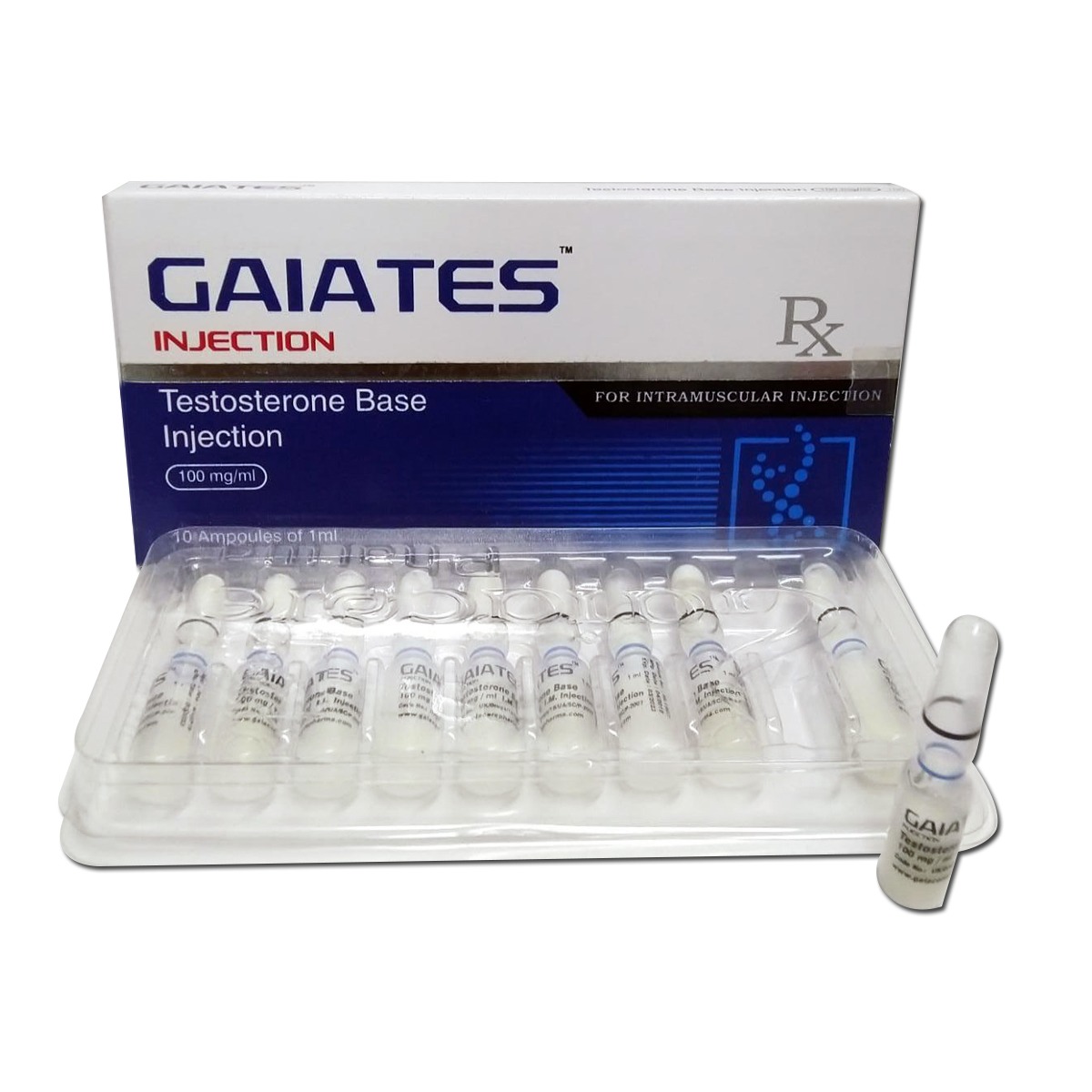
Gaiates
Composition:
Each ml contains: Testosterone Base 100 mg
Dosage:
For dosage, administration & detailed direction for use, see package insert. Laboratory Tested & Quality Guaranteed. This product is produced under GMP Pharmaceuticals Environment.
Pharmacological Action:
Gaiates is an injectable anabolic preparation. After injection, testosterone base is gradually released from the intramuscular depot and subsequently hydrolysed into nandrolone.
Indications:
Certain cases of disseminated breast cancer in women. Osteoporosis due to androgen deficiency in hypogonadal males.
Precautions:
Virilisation which appears in sensitive women as hoarseness, acne, hirsutism, and increased libido; in prepubertal boys as an increased frequency of erections and phallic enlargement, and in girls as an increase of pubic hair and clitoral hypertrophy. Hoarseness may be the first symptom of vocal change which may end in a long-lasting, sometimes irreversible deepening of the voice.








.jpg)